Group Properties
We saw that elements located in the same column of the periodic table are classified as a group. Elements in the same group share many similarities. They also show differences that tend to follow a pattern as you move up or down within a group. To understand why these patterns occur, we need to explore the arrangement of electrons in atoms.
Electron Arrangements in Atoms
You have learned that the electrons in an atom occupy energy levels and that the pattern of electrons filling energy levels gives the electron configuration for that atom. Take a look at the three elements in Table 3. Each of these elements belongs to group 1 in the periodic table. Notice that as you move down the group from period to period, the atomic number and atomic mass of the atoms become larger as they gain protons and electrons. This is an important difference between elements within the same group.
Electron dot structures (also known as Lewis structures) combine the chemical symbol for an element and dots to show the element’s valence electrons. Notice that all of the elements in group 1 have one valence electron, as shown by the electron dot structures in the right-hand column.
Table 3: Some group 1 elements and their electron dot structures
If you look at the valence electrons in other groups of the periodic table, you will find that they also share similar patterns. The periodic table below shows the Lewis dot symbols for some elements. Notice that all elements in any group share the same valence electron configuration, except for transition metals and helium.
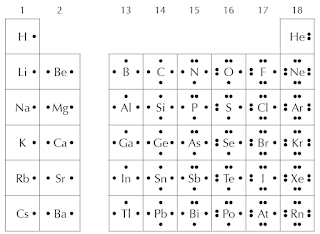 |
Figure 24 A summary of electron dot structures for elements in groups 1 and 2,
and groups 13 through 18, of the periodic table. |
Which of the following statements is/are true about atoms of sulfur (S) and selenium (Se)?
Selenium has similar chemical reactivity as sulfur.
Selenium has a larger mass than sulfur.
Selenium is chemically more reactive than sulfur.
Selenium and sulfur have the same mass.
answer :
Selenium has a larger mass than sulfur.
|
Selenium has similar chemical reactivity as sulfur.
Or check the whole part
1.3 Group properties |


