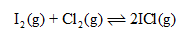Question 1
Not yet answered
Marked out of 1.00
Flag question
Question textPAr
Part 1
Choose the correct answers to complete the following.
a. Equilibrium can exist only when all particles are kept in a(n) container and certain conditions are kept .
b. A dynamic equilibrium is reached when the forward and backward reactions occur at .
c. The macroscopic properties of a system at equilibrium are .
Question 2
Not yet answered
Marked out of 1.00
Flag question
Question text
Consider the following reaction.
Choose the correct answers.
a. When the temperature of this reaction is increased, the equilibrium concentration of Cl2 will .
b. When the equilibrium concentration of O2 is increased, the equilibrium concentration of Cl2 will .
c. When a catalyst is added to the system, the equilibrium concentration of Cl2 will
Question 3
Not yet answered
Marked out of 1.00
Flag question
Question 4
Not yet answered
Marked out of 1.00
Flag question
Question 5
Not yet answered
Marked out of 1.00
Flag question
Question 6
Not yet answered
Marked out of 1.00
Flag question
Question text
Which of the following affect the equilibrium of a reaction? Select all that apply.
Select one or more:
Question 7
Not yet answered
Marked out of 1.00
Flag question
Question text
Few drops Fe(NO3)3 are added to a solution containing KSCN and water.
Consider the following reaction.
What would happen if few crystals of Na2HPO4 are added to the mixture at equilibrium?
[Hint: The red color of the mixture is due to the hydrated thiocyanoiron(III) ion, FeSCN2+.]
Select one or more:
Question 8
Not yet answered
Marked out of 1.00
Flag question
Question text
Match each of the following systems to identify them as open or closed.
a. a Bunsen burner flame:
b. a sealed bottle of sparkling water:
c. a cup of soda:
Question 9
Not yet answered
Marked out of 1.00
Flag question
Part 2
Question 2
Not yet answered
Marked out of 1.00
Flag question
Question text
Hydrogen and nitrogen react to form ammonia as shown in the following chemical equation.
Values of the equilibrium constant at various temperatures are given below.
Keq (at 298 K) = 3 × 108
Keq (at 450 K) = 3 × 103
Keq (at 600 K) = 4.1
At which temperature the system will contain the highest proportion of hydrogen and nitrogen in the equilibrium mixture?
K
Question 3
Not yet answered
Marked out of 1.00
Flag question
Question text
The following reaction is the reaction of synthesis of ammonia at 500°C.
The equilibrium concentrations of the species present are recorded as follows.
[N2] = 1.50 × 10−5 M; [H2] = 3.54 × 10−1 M; [NH3] = 2.00 × 10−4 M
Calculate the value of the equilibrium constant, Keq at 500°C of the above reaction.
Give your answer to three significant figures.
K =
Question 4
Not yet answered
Marked out of 1.00
Flag question
Question text
Choose the correct answers.
The equilibrium tends to move in the direction of the potential energy and the randomness.
Question 5
Not yet answered
Marked out of 1.00
Flag question
Question text
Given the following reaction.
Choose the correct answer to predict the position of the equilibrium under the following conditions.
a. The concentrations of the gases are:
[N2] = 0.04 M; [O2] = 0.1 M; [NO] = 0.2 M
The system is .
b. The concentrations of the gases are:
[N2] = 0.4 M; [O2] = 0.1 M; [NO] = 0.005 M
The system is .
c. The concentrations of the gases are:
[N2] = 0.04 M; [O2] = 0.1 M; [NO] = 0.002 M
The system is .
Question 6
Not yet answered
Marked out of 1.00
Flag question
Question text
The equilibrium constant, Keq, of the following reaction is 5.0 at a certain temperature.
The same number of moles of each of I2 and Cl2, was injected into a 2.0 L container. The equilibrium concentration of ICl is found to be 0.10 M.
a. Calculate the concentration of Cl2 and I2 at equilibrium.
Give your answers to two significant figures.
[I2] = M
[Cl2] = M
b. Find the initial concentration of Cl2 and I2.
Give your answer to two significant figures.
[I2] = M
[Cl2] = M
c. Find the initial number of moles of Cl2 and I2.
n of I2 = mol
n of Cl2 = mol
Question 7
Not yet answered
Marked out of 1.00
Flag question
Question 8
Not yet answered
Marked out of 1.00
Flag question
Question 9
Not yet answered
Marked out of 1.00
Flag question
Question 10
Not yet answered
Marked out of 1.00
Flag question
Drag and drop from the box below to complete the following.
a. Equilibrium can exist only when all particles are kept in a(n) blank container and certain conditions are kept blank .
b. A dynamic equilibrium is reached when the forward and backward reactions occur at blank .
c. The macroscopic properties of a system at equilibrium are blank
SEALED - OPEN - CONSTANT - DIFFERENT RATES - THE SAME RATE -VARIABLE
The correct answer is:
a. Equilibrium can exist only when all particles are kept in a(n) [sealed ] container and certain conditions are kept [constant].
b. A dynamic equilibrium is reached when the forward and backward reactions occur at [the same rate].
c. The macroscopic properties of a system at equilibrium are [constant].
Consider the following reaction.
Drag and drop to complete the following.
a. When the temperature of this reaction is increased, the equilibrium concentration of Cl2 will blank .
b. When the equilibrium concentration of O2 is increased, the equilibrium concentration of Cl2 will blank .
c. When a catalyst is added to the system, the equilibrium concentration of Cl2 will blank .
The correct answer is:
Consider the following reaction.
a. When the temperature of this reaction is increased, the equilibrium concentration of Cl2 will [decrease].
b. When the equilibrium concentration of O2 is increased, the equilibrium concentration of Cl2 will [increase].
c. When a catalyst is added to the system, the equilibrium concentration of Cl2 will [remain constant].
Consider the following reaction.
Choose the correct answer to complete the sentence below.
If the equilibrium concentration of Cl2 is increased, the equilibrium concentration of COCl2 will increase or decrease .
Feedback
The correct answer is:




Consider the following reaction.
Choose the correct answer to complete the sentence below.
If the equilibrium concentration of Cl2 is increased, the equilibrium concentration of COCl2 will [increase].
Consider the following reaction.
The total pressure is increased by injecting helium gas into the chamber. Which of the following is true about the reaction?
Select one:
Feedback
The correct answer is: The equilibrium [HI], [H2] and [Cl2] are not affected.
Consider the following reaction.
The partial pressure of H2 is decreased.
Which of the following is true about the reaction?
Select one or more:
Feedback
The correct answers are: The equilibrium [H2O] will decrease., The equilibrium concentration of [O2] will increase.
Which of the following affect the equilibrium of a reaction? Select all that apply.
Select one or more:
Feedback
The correct answers are: changing the temperature, changing the concentration of the reactants or products
Few drops Fe(NO3)3 are added to a solution containing KSCN and water.
Consider the following reaction.
What would happen if few crystals of Na2HPO4 are added to the mixture at equilibrium?
[Hint: The red color of the mixture is due to the hydrated thiocyanoiron(III) ion, FeSCN2+.]
Select one or more:
Feedback
The correct answers are: [Fe3+] will decrease., The red color diminishes.
Match each of the following systems to identify them as open or closed.
a. a Bunsen burner flame:
b. a sealed bottle of sparkling water:
c. a cup of soda:
Feedback
The correct answer is:


Match each of the following systems to identify them as open or closed.
a. a Bunsen burner flame: [open system]
b. a sealed bottle of sparkling water: [closed system]
c. a cup of soda: [open system]
Consider the following reaction.
Choose the correct answer to complete the sentence below.
When the temperature is decreased, the equilibrium . The equilibrium [N2O4] .
The correct answer is:
Consider the following reaction.
Choose the correct answer to complete the sentence below.
When the temperature is decreased, the equilibrium [shifts to the left]. The equilibrium [N2O4] [increases].