Chemical Equations
Have you ever used social media to chat with a friend? If so, you are probably familiar with some of well-known abbreviations used in our daily life to communicate more quickly with friends. For example, you might be able to read the following message: “bfn gtg ttyl tc n gn.”(bye for now got to go talk to you later take care and good night.).
In a similar way, chemists draw representations of chemical reactions. Instead of writing a whole sentence, chemists use symbols to represent the beginning substances and the ending substances separated by an arrow to represent the changes in a chemical reaction.
Figure 10 Just as people use abbreviations and emoticons in social media to quickly communicate their ideas,
a chemist use symbols and arrows to represent a chemical reaction.
a chemist use symbols and arrows to represent a chemical reaction.
Representing Chemical Reactions Using Balanced Chemical Equations
Word equations are very descriptive, but they do not communicate as much information as equations written using chemical formulas. Take the reaction between methane and oxygen as an example. The equation using chemical formulas, shown below, gives several pieces of information that the word equation does not.
This equation shows the chemical formulas as well as the number of molecules of each reactant and product involved in this reaction. The number of molecules participating in the chemical reaction is indicated to the left of each chemical formula in the equation. This number is called a coefficient. The coefficients in a chemical equation tell you how many molecules of each reactant are present before the reaction occurs and how many molecules of each product are formed. When no coefficient is present, the coefficient is “1.” In this case, one CH4 molecule reacts with two O2 molecules to form one CO2 molecule and two H2O molecules. The same numbers of atoms of each element are represented on each side of the equation.
The coefficients tell us how many molecules of reactants and products are involved in a chemical reaction. But what about the numbers of atoms that make up the molecules? We already know that in a chemical formula, the subscripts indicate the number of atoms of each element in a particular molecule. If there is no subscript, it indicates there is one atom. To find the number of atoms of an element involved in a reaction, multiply the coefficient by the subscript of the atom. For example, in the reactants of a methane – oxygen reaction, there is 1 atom of carbon (1×1), 4 atoms of hydrogen (1×4), and 4 atoms of oxygen (2×2).
Indicating States of Matter in Chemical Equations
It is often useful to know the state of each reactant and product in a chemical reaction. For example, if two solutions react to produce a solid product that separates from the solution, this product is called a precipitate and the reaction is called a precipitation reaction. Because there are often two products in this kind of reaction and only one is a precipitate, it is useful to know which product is the precipitate. Chemists indicate the precipitate using the symbol (s) to the right of the chemical formula in the chemical equation. The (s) symbol stands for solid. In addition to solids, other states are indicated with labels. Gases are indicated with the symbol (g) and liquids with the symbol (l).
Some chemists use the symbols ↓ to represent a precipitate and ↑ to represent a gas. The symbol (aq) is used to indicate that the reactant or product is present in an aqueous solution. An aqueous solution is one that uses water as the solvent. The following equation shows an example of a reaction that produces one product that precipitates as a solid.
It is important to note that when liquid water is a reactant or product, it is given the state symbol (l). For example, the reaction between sodium metal and water to form sodium hydroxide solution and hydrogen is represented by the equation:
Atoms of Products Equal Atoms of Reactants
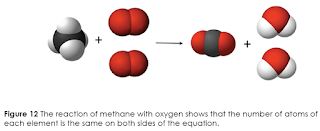
The atoms present before a reaction do not change in quantity or identity as they undergo rearrangement during a reaction. Molecules, on the other hand, may change in both identity and number. You can use the chemical equation and its coefficients to show that no atoms are gained or lost during a reaction. The reaction of methane with oxygen serves as an example
This reaction can be represented as models, as shown below. The black balls represent carbon atoms, the white balls represent hydrogen atoms, and the red balls represent oxygen atoms.
To see how atoms are conserved, count the number of atoms for each element on the reactant side and then make the same count on the product side of the equation. The results are shown in the table below.
These results show the same number of atoms of each element in the reactants and in the products. The number of atoms does not change during the chemical reaction. The bonds holding the atoms together are broken, which allows the atoms to rearrange and form new bonds in the products.
The chemical equation for this reaction demonstrates that the number and type of atoms in the reactants are the same as those in the products. Such a chemical equation is balanced. A balanced chemical equation is an equation in which the number of each type of atom is the same on both sides of the arrow.
Mass of Products Equals Mass of Reactants
You saw earlier how the number of atoms of each element is conserved during a chemical reaction. You can also determine whether mass is also conserved. Consider the chemical reaction between methane and oxygen analyzed earlier as an example:
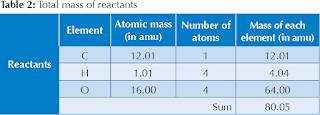
To compare masses of reactants and products, we find the atomic masses of the elements present on the reactant side of the equation and we list them in a table. Then, we calculate the total mass of each element on the reactants side by multiplying the atomic mass of each element by the number of atoms of that element. Finally, we add all of the masses to determine the combined mass of the reactants. The results are shown in the table below.
Next, the same analysis is carried out for the product side of the equation. After listing all of the elements, their atomic masses, and number of atoms of each element in a table, the total mass of each element is calculated. Finally, we find the combined mass of the products. The table below shows the results.
As you can see from this analysis, the mass remains constant during a chemical reaction because the same atoms are present before and after the reaction. The only difference is that the atoms have been rearranged into new bonding patterns in the products. Just as numbers of atoms are conserved during a chemical reaction, mass is also conserved. The combined mass of the reactants is the same as the combined mass of the products.
These findings demonstrate the law of conservation of mass, which states that mass does not change as reactants are converted to products in a chemical reaction. You may also see this law stated as: Mass (or matter) is neither created nor destroyed during chemical processes.