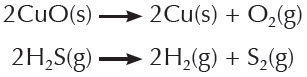In terms of chemistry, what do a burning candle, a speeding car, and a sleeping kitten have in common?
A fourth group of reactions includes the replacement reactions. In a replacement reaction, one or two elements replace other elements in compounds. If one element replaces another in a compound, the reaction is a single-replacement reaction. If two elements in two compounds change places to replace each other, the reaction is a double-replacement reaction. Note that, in addition to elements, polyatomic ions composed of groups of atoms may also be exchanged. Common examples are nitrate ion, NO3–, and ammonium ion, NH4+.
We will look more closely at single-replacement reactions first and double-replacement reactions a bit later. The basic pattern of a single replacement reaction is shown below.
An example of a single-replacement reaction is the reaction between iron and lead nitrate:
Note that iron (Fe) replaces lead (Pb) in the reaction.
In a single-replacement reaction, one element replaces another element to form a new compound. In a double-replacement reaction, two elements in two compounds replace each other to form two new compounds. The basic pattern of a double-replacement reaction is shown below.
An example of a double-replacement reaction is the reaction between silver nitrate and sodium chloride:
In this reaction, silver ion (Ag+) and sodium ion (Na+) replace each other by trading places. It could also be said that NO3– and Cl– replace each other.
Al2(CO3)3 → Al2O3 + 3CO2
a combustion reaction
a decomposition reaction
a synthesis reaction
a single-replacement reaction
a double-replacement reaction
answer :
a decomposition reaction
Response:
That's the correct answer
What is the type of the reaction below?
Mg + 2AgNO3 → 2Ag + Mg(NO3)2
a single-replacement reaction
a double-replacement reaction
a synthesis reaction
a decomposition reaction
a combustion reaction
Mg + 2AgNO3 → 2Ag + Mg(NO3)2
a single-replacement reaction
a double-replacement reaction
a synthesis reaction
a decomposition reaction
a combustion reaction
End of Chapter 3
Go to main menu
End of topic