The Mole Concept
Bookshops usually sell different types and sizes of paper for printers and copiers in packages. Each pack contains a specific number of identical papers. For example, a rim of A4 papers usually contains 500 papers. By assigning a price to each rim, the seller would be able to ease the buying and selling process.
In a similar way, chemists group atoms or molecules into units of known quantities. This helps them keep track of large numbers of atoms and molecules as well as easily determine how many particles are in a sample at any time.
Avogadro’s Number
According to Avogadro’s hypothesis, we can tell a lot about a gas by just knowing the number of its particles, even when we don’t know its identity. For example, equal numbers of particles of gases, such as neon, nitrogen, water vapor, and ammonia, all have the same volume when they are at the same temperature and pressure.
Figure 14 Equal volumes of gases at the same temperature and pressure have the same number of gas particles.
Avogadro had no way to determine the number of particles in a gas, and therefore no firm evidence to support his hypothesis. He constructed his hypothesis using logic and reasoning in conjunction with Gay-Lussac’s observations of the way gas volumes combined in whole number ratios.
At first, scientists rejected Avogadro’s hypothesis. Nine years after Avogadro’s death, an Austrian scientist named Johann Josef Loschmidt used laboratory methods to determine the number of particles in a volume of gas. This achievement enabled Avogadro’s hypothesis to finally be confirmed through experiment.
Today, chemists use sophisticated lab equipment to determine the number of atoms and molecules in substances. It is often important to compare large numbers of particles in order to predict the outcome of chemical reactions. A standard unit was needed for such comparisons. It was decided to use the number of carbon atoms in a 12-gram sample of the isotope carbon-12. That number is 602,213,670,000,000,000,000,000 atoms; it is called Avogadro’s number in honor of Avogadro’s pioneering work with particles. Because it is such a large number, it is expressed in scientific notation with four significant figures, which will be enough for the calculations we perform. Thus, Avogadro’s number is expressed as
NA = 6.022 × 1023
What mass of what isotope is used to standardize Avogadro’s number?
12 grams of carbon-12 are used.
12 grams of hydrogen-1 are used.
10 grams of carbon-12 are used.
15 grams of hydrogen-1 are used.
answer
12 grams of carbon-12 are used.
What mass of what isotope is used to standardize Avogadro’s number?
he Mole
Certain numbers are given names. For example, the number 12 is called a dozen, and the number 1.0 × 106 is called a million. In chemistry, the number 6.022 × 1023 is called a mole. One mole of atoms consists of 6.022 × 1023 atoms; similarly, one mole of ions consists of 6.022 × 1023 ions.
Generally, chemists use Avogadro’s number when dealing with atoms, molecules, ions, electrons, protons, or neutrons.
You can use Avogadro’s number to determine the number of particles in a sample. The process is the same as the one you use to find the number of eggs in 5 dozens of eggs. You know that you have 12 eggs per dozen and that you have 5 dozens, so you multiply 12 × 5 to find a total of 60 eggs. Similarly, knowing that there are 6.022 × 1023 protons per mole; therefore, 3 moles of protons contain 3 × 6.022 × 1023 = 1.807 × 1024 protons. The calculation is shown in greater detail below.
You can also carry out calculations to find the number of moles of a substance if you know how many particles of that substance you have. In this case, divide the number of particles by Avogadro’s number to find the number of moles. Let us say that you have 3.56 × 1023 molecules of water. To find how many moles of water you have, set up a calculation in which you divide by Avogadro’s number as shown in the calculation below.
Notice that moles are not restricted to whole-number values and that you can have numbers of moles that are smaller than 1. In addition, when used as a unit, a mole is often abbreviated “mol.” For example, 1.88 mol of barium sulfate is a description of a sample of barium sulfate comprised of 1.88 moles.
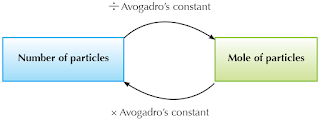
It is also important to understand that mole calculations apply to any type of particle in the same way. In other words, there is no difference in the way that you calculate moles of atoms, molecules, electrons, protons, or neutrons. Apply Avogadro’s constant as shown in the figure below to determine the number of moles of any type of particle.
Figure 16 Avogadro’s number can be used to convert between moles and number of particles.
Which of the following statements is true?
Which of the following statements is true?
6.022 × 1023 is Avogadro’s hypothesis and is called a mole.
6.022 × 10−23 is Avogadro’s hypothesis and is called a mole.
6.022 × 10−23 units is Avogadro’s number and is called a mole.
6.022 × 1023 units is Avogadro’s number and is called a mole.
6.022 × 1023 units is Avogadro’s number and is called a mole.
Fill in the blank.
In 0.20 mol of He there is 1.2 × 10b atoms. What is b?
[He = 4.0; NA = 6.0 × 10−23]
b =
Next Part
Back to the Main Menu
Bookshops usually sell different types and sizes of paper for printers and copiers in packages. Each pack contains a specific number of identical papers. For example, a rim of A4 papers usually contains 500 papers. By assigning a price to each rim, the seller would be able to ease the buying and selling process.
In a similar way, chemists group atoms or molecules into units of known quantities. This helps them keep track of large numbers of atoms and molecules as well as easily determine how many particles are in a sample at any time.
Avogadro’s Number
According to Avogadro’s hypothesis, we can tell a lot about a gas by just knowing the number of its particles, even when we don’t know its identity. For example, equal numbers of particles of gases, such as neon, nitrogen, water vapor, and ammonia, all have the same volume when they are at the same temperature and pressure.
Figure 14 Equal volumes of gases at the same temperature and pressure have the same number of gas particles.
Avogadro had no way to determine the number of particles in a gas, and therefore no firm evidence to support his hypothesis. He constructed his hypothesis using logic and reasoning in conjunction with Gay-Lussac’s observations of the way gas volumes combined in whole number ratios.
At first, scientists rejected Avogadro’s hypothesis. Nine years after Avogadro’s death, an Austrian scientist named Johann Josef Loschmidt used laboratory methods to determine the number of particles in a volume of gas. This achievement enabled Avogadro’s hypothesis to finally be confirmed through experiment.
Today, chemists use sophisticated lab equipment to determine the number of atoms and molecules in substances. It is often important to compare large numbers of particles in order to predict the outcome of chemical reactions. A standard unit was needed for such comparisons. It was decided to use the number of carbon atoms in a 12-gram sample of the isotope carbon-12. That number is 602,213,670,000,000,000,000,000 atoms; it is called Avogadro’s number in honor of Avogadro’s pioneering work with particles. Because it is such a large number, it is expressed in scientific notation with four significant figures, which will be enough for the calculations we perform. Thus, Avogadro’s number is expressed as
NA = 6.022 × 1023
What mass of what isotope is used to standardize Avogadro’s number?
12 grams of carbon-12 are used.
12 grams of hydrogen-1 are used.
10 grams of carbon-12 are used.
15 grams of hydrogen-1 are used.
answer
12 grams of carbon-12 are used.
What mass of what isotope is used to standardize Avogadro’s number?
he Mole
Certain numbers are given names. For example, the number 12 is called a dozen, and the number 1.0 × 106 is called a million. In chemistry, the number 6.022 × 1023 is called a mole. One mole of atoms consists of 6.022 × 1023 atoms; similarly, one mole of ions consists of 6.022 × 1023 ions.
Generally, chemists use Avogadro’s number when dealing with atoms, molecules, ions, electrons, protons, or neutrons.
You can use Avogadro’s number to determine the number of particles in a sample. The process is the same as the one you use to find the number of eggs in 5 dozens of eggs. You know that you have 12 eggs per dozen and that you have 5 dozens, so you multiply 12 × 5 to find a total of 60 eggs. Similarly, knowing that there are 6.022 × 1023 protons per mole; therefore, 3 moles of protons contain 3 × 6.022 × 1023 = 1.807 × 1024 protons. The calculation is shown in greater detail below.
You can also carry out calculations to find the number of moles of a substance if you know how many particles of that substance you have. In this case, divide the number of particles by Avogadro’s number to find the number of moles. Let us say that you have 3.56 × 1023 molecules of water. To find how many moles of water you have, set up a calculation in which you divide by Avogadro’s number as shown in the calculation below.
Notice that moles are not restricted to whole-number values and that you can have numbers of moles that are smaller than 1. In addition, when used as a unit, a mole is often abbreviated “mol.” For example, 1.88 mol of barium sulfate is a description of a sample of barium sulfate comprised of 1.88 moles.
It is also important to understand that mole calculations apply to any type of particle in the same way. In other words, there is no difference in the way that you calculate moles of atoms, molecules, electrons, protons, or neutrons. Apply Avogadro’s constant as shown in the figure below to determine the number of moles of any type of particle.
Figure 16 Avogadro’s number can be used to convert between moles and number of particles.
Which of the following statements is true?
Which of the following statements is true?
6.022 × 1023 is Avogadro’s hypothesis and is called a mole.
6.022 × 10−23 is Avogadro’s hypothesis and is called a mole.
6.022 × 10−23 units is Avogadro’s number and is called a mole.
6.022 × 1023 units is Avogadro’s number and is called a mole.
6.022 × 1023 units is Avogadro’s number and is called a mole.
Fill in the blank.
In 0.20 mol of He there is 1.2 × 10b atoms. What is b?
[He = 4.0; NA = 6.0 × 10−23]
b =
Atomic Mass Units
Atoms are extremely small, which means that their masses are extremely small also. For this reason, the gram is not a convenient unit for describing the mass of an atom. This is why scientists introduced a new unit of mass, the atomic mass unit, amu. The atomic mass unit is defined such that 1 amu is exactly 1/12 of the mass of a carbon-12 atom, or such that 1 atom of carbon-12 has a mass of 12 amu:
Mass of 1 atom of carbon-12 = 12 amu
If you look at the periodic table, you will see that the atomic mass for carbon is 12.01. You can use the atomic mass for any element to find the mass of one atom of that element in amu.
Figure 18 The atomic mass of any element listed on the periodic table is numerically equal
to the mass in atomic mass units of one atom of that element.
Note that the relative atomic masses listed in the periodic table are unitless numbers. This is because each atomic mass has been determined relative to the carbon-12 standard, which was assigned an atomic mass of 12 amu. Thus, the mass of one atom of fluorine is calculated using the relative atomic masses from the periodic table multiplied by the standard atomic mass of carbon-12:
× 12 amu =19 amu
It is also important to note that the atomic masses listed on the periodic table represent averages based on the natural abundances of isotopes of each element. In the case of carbon atoms, there are three naturally occurring isotopes: carbon-12, carbon-13, and carbon-14. If you take a natural sample containing these isotopes, you find that the average mass of the collection of carbon atoms is 12.01. For most situations you will encounter, all elements will reflect these natural abundances, and so you should use these average atomic masses for your calculations. For example, use the atomic mass of 12.01 amu for the mass of one atom of carbon. Just remember that no single atom of carbon actually has the mass 12.01, and that this mass represents the average mass of the naturally occurring isotopes of carbon.
Recall that the mass of one proton is almost equal to the mass of one neutron, and both have a mass very close to 1.0 amu. For example, carbon-12 is made up of 6 protons and 6 neutrons, which conforms with the agreed upon carbon-12 mass of 12 amu. Likewise, fluorine has 9 protons and 10 neutrons for a total of 19 amu. (Fluorine-19 is the only natural isotope of fluorine.)
What is the mass of one atom of sodium, 2311Na?
11 g
23 amu
11 amu
23 g
answer 23 amu
Converting between Grams and Atomic Mass Units
Grams and atomic mass units (amu) are units of mass. Because both units measure the same property, you can convert one unit to the other using a conversion factor. To establish the relationship between the gram and the amu, you can start with one mole of carbon-12 atoms. One mole of carbon-12 atoms has a mass of 12 grams.
Mass of 1 mole of carbon-12 = 12 grams
You also know that one mole of carbon-12 atoms contains 6.022 × 1023 atoms. Each atom has a mass of 12 amu. Therefore, the mole of carbon-12 atoms has the following mass:
Mass of 1 mole of carbon-12 = 6.022 × 1023 × 12 amu
Both equations give the mass of one mole of carbon-12 atoms, so we can write the following to equate the two:
12 grams = 6.022 × 1023 × 12 amu
Divide both sides of this equation by 12 to simplify to the following:
1.000 g = 6.022 × 1023 amu
From this relationship, two conversion factors can be written:
and
These conversion factors can be used for converting one unit to the other. For example, you can calculate the mass in grams of 100 copper atoms. You cannot actually measure such a small mass, but you can determine the mass through calculations. Begin by finding the atomic mass of copper on the periodic table. The atomic mass of any element is then used to give the mass of one atom of that element in amu. For copper, the mass of one copper atom is 63.55 amu. Multiply this mass by the number of copper atoms in the sample to find the mass of the entire sample:
Next, use the relationship between amu and grams as a conversion factor to multiply times the mass in amu of the copper sample. This will give you the mass in grams of 100 atoms of copper:
11 g
23 amu
11 amu
23 g
answer 23 amu
Converting between Grams and Atomic Mass Units
Grams and atomic mass units (amu) are units of mass. Because both units measure the same property, you can convert one unit to the other using a conversion factor. To establish the relationship between the gram and the amu, you can start with one mole of carbon-12 atoms. One mole of carbon-12 atoms has a mass of 12 grams.
Mass of 1 mole of carbon-12 = 12 grams
You also know that one mole of carbon-12 atoms contains 6.022 × 1023 atoms. Each atom has a mass of 12 amu. Therefore, the mole of carbon-12 atoms has the following mass:
Mass of 1 mole of carbon-12 = 6.022 × 1023 × 12 amu
Both equations give the mass of one mole of carbon-12 atoms, so we can write the following to equate the two:
12 grams = 6.022 × 1023 × 12 amu
Divide both sides of this equation by 12 to simplify to the following:
1.000 g = 6.022 × 1023 amu
From this relationship, two conversion factors can be written:
and
These conversion factors can be used for converting one unit to the other. For example, you can calculate the mass in grams of 100 copper atoms. You cannot actually measure such a small mass, but you can determine the mass through calculations. Begin by finding the atomic mass of copper on the periodic table. The atomic mass of any element is then used to give the mass of one atom of that element in amu. For copper, the mass of one copper atom is 63.55 amu. Multiply this mass by the number of copper atoms in the sample to find the mass of the entire sample:
Next, use the relationship between amu and grams as a conversion factor to multiply times the mass in amu of the copper sample. This will give you the mass in grams of 100 atoms of copper:
Measuring Moles Using Mass
You can find the mass of one mole of atoms by multiplying Avogadro’s number by the mass of one atom:
Mass of 1 mole of atoms = 6.022 × 1023 × mass of 1 atom
For example, the mass of one mole of oxygen atoms is:
(6.022 × 1023 × 16 amu)
Using the conversion factor to convert amu to grams, you can find this mass in grams:
Look more closely at this result. The calculation shows that one mole of oxygen atoms has a mass of 16.00 grams. The number 16.00 is the same value of the mass in atomic mass units (amu) of one oxygen atom. Therefore, chemists make the generalization that a mole of any element has a mass in grams numerically equal to the mass in amu of one atom of that element. In other words, one mole of an element is its atomic mass expressed in grams.
The mass-mole relationship is a convenient tool for chemists. It allows a chemist to look up the atomic mass for an element on the periodic table and immediately use that number as the mass in grams of one mole of atoms of that element. For example, according to the periodic table, one mole of hydrogen atoms has a mass of 1.008 grams, one mole of helium atoms has a mass of 4.003 grams, and one mole of lithium atoms has a mass of 6.941 grams.
Figure 19 A balance or scale can be used to measure out the mass of
a substance that contains a mole of particles.
Atomicity
When the smallest unit of a pure substance is a molecule, it is important to consider all of the atoms present in a single molecule. Chemists use the term atomicity to refer to the number of atoms present in a molecule. Thus, the atomicity of a molecule of O2 is two. The atomicity of a molecule of water, H2O, is three.
The atomicity of molecular substances must be considered when making quantitative calculations involving masses and moles. For example, suppose that a chemist has 3.0 moles of oxygen gas. This means that there are 3.0 moles of O2 molecules, but 6.0 moles of oxygen atoms. From the periodic table, you can find the atomic mass of oxygen, which is 16.00. Since this is the mass of one atom of oxygen, the mass of one molecule of O2 is 16.00 × 2 or 32.00 amu. This is the mass of one molecule of O2. The mass of a molecule is the sum of the masses of its atoms.
m = 108 g
answer 7
answer 28
Next Part
Back to the Main Menu