Network Solids
The covalent compounds you have studied above consist of individual molecules. However, there are other covalent compounds that are classified as network solids, rather than as molecules. A network solid consists of a large number of atoms covalently bonded together, forming a three-dimensional network.
Carbon atoms can bond together to form two well-known network solids, diamond and graphite. In both cases, there is no limit to the number of atoms that could theoretically be present. Each network solid is capable of being extended far beyond the structures shown in Figures 27 and 28.
These figures represent part of the structures of diamond and graphite. Each dot represents a single carbon atom, and each line represents a covalent bond between two carbon atoms. Notice that the atoms in diamond form a cage-like structure in which each carbon atom is covalently bonded to four other carbon atoms. This structure is repeated to form the network solid. Diamonds are extremely hard solids—so hard that they are used to cut steel.
Graphite is much softer than diamond and is used as a lubricant and as pencil “lead.” The arrangement of carbon atoms in graphite is shown in the figure below. The dashed lines represent attractive, non-covalent forces between the covalently bonded layers. The layers are made up of repeating six-member carbon rings. Each carbon atom in graphite is bonded to three other carbon atoms. The leftover electrons are delocalized around each ring.
Although diamond and graphite have the same elemental composition, they have very different physical properties. Some of these properties are described in Table 1. The differences are due to differences in their network solid structures.
The repeating cage-like lattice structure in diamond is responsible for its properties. All of the carbon atoms throughout the lattice are covalently bonded to one another, which makes diamond very strong and difficult to break and accounts for its very hard nature. The repeating, regular spacing of the carbon atoms allows the structure to have very smooth sides as well as a transparent and colorless appearance.
Graphite’s structure can also be used to explain its properties. Strong covalent bonds are only present in two of the three dimensions. The noncovalent forces of attraction in the other dimension are not strong enough to prevent sliding of the covalently bonded layers of carbon atoms. As a result, graphite is much softer than diamond and has a slippery texture. Its black color results from the fact that the flat sheets of carbon can absorb light, while in diamond the cage-like structure cannot. The delocalized electrons in the graphite’s structure also enable it to transmit electric current, in contrast to diamond, which does not.
answer :
all parts have an identical structure.
|
| Response: That's the correct answer |
answer :
atoms form a cage-like structure
= Diamond
atoms form a flat sheet structure
= Graphite |
| Response: That's the correct answer |
4
answer :
Diamond and graphite have different physical properties because of differences in their
= structures
The carbon atoms are all bonded in one continuous, three-dimensional network structure so that the entire structure is rigid in
= diamond
The carbon atoms are bonded in flat sheets stacked on one another in
= graphite |
| Response: That's the correct answer |
Properties of Covalent Compounds - Cont.
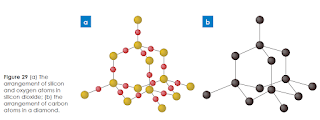
A third network solid is silicon dioxide. The left side of Figure 29 represents the arrangement of atoms found in silicon dioxide, which contains silicon atoms shown in yellowish brown and oxygen atoms shown in red. The structure to the right represents the arrangement of atoms found in diamond, which contains carbon atoms shown in black.
Silicon dioxide is also known as the mineral quartz. Like diamond, pure quartz is colorless and transparent. Quartz is not as hard as diamond but it is much harder than graphite.
The melting point of quartz is also significantly lower than that of diamond, although it is still a very high melting point in comparison with many other solids. Quartz also has a lower density than diamond, which indicates that the atoms are not as tightly packed in quartz as they are in diamond. Finally, neither diamond nor quartz are conductors of electric current. They share this property because all their valence electrons are held tightly in fixed bonds.
Which of the following statements is true?
Diamond and graphite have similar physical properties because both are made up of carbon atoms.
The arrangement of atoms in a network solid does not affect the properties of the substance.
Diamond and silicon dioxide have similar physical properties because their atoms are arranged in a cage-like structure.
Diamond and graphite have different physical properties because they have different structures.
9
10
Trends in Science
Both graphite and diamond are naturally occurring network solids. Recently, scientists have synthesized a new class of carbon-based network solids called fullerenes. The first fullerenes to be made were buckyballs. A buckyball consists of closed spheres containing 20 to 80 carbon atoms. Each carbon atom is bonded to three others to form rings of either five or six carbon atoms. Like graphite, some delocalized electrons belong to each ring. The rings are interconnected to form a large closed sphere that looks like the pattern of seams on a soccer ball. Scientists are finding ways to use buckyballs as carriers for drugs and other small molecules.
A buckyball is composed of repeating carbon rings.
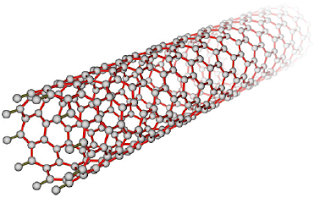
Since buckyballs were discovered, another set of fullerene structures called carbon nanotubes have been synthesized. As you might guess from their name, carbon nanotubes are long, hollow cylinders of interconnected carbon atoms. Because they can be made in different lengths and tube diameters, carbon nanotubes have the potential to be made to suit specific applications such as drug delivery and cancer therapy. They also conduct electric current, which allows them to be used in miniature electrical devices.
A nanotube is a type of fullerene.
- Would you expect a sample containing millions of molecules of a fullerene to exhibit properties more like those of graphite or of diamond? Explain.
- Carbon nanotubes have been shown to be one of the strongest human-made materials known. What structural properties of carbon nanotubes explain this amazing strength?
End of topic
Go back to Chemical bonding other topics
Go back to Chemical bonding other topics