1.2.1 Names and uses of chemical apparatus
- Pipette: To measure specific amounts of liquid (e.g. exactly 10 cm3, exactly 25 cm3, etc., to the nearest 0.05 cm3).
- Burette: To measure variable volumes of liquids from 0 to 50 cm3 e.g. 16.7 cm3; to the nearest 0.05 cm3 per reading or 0.1 cm3 per sample taken, since determining the volume of liquid used requires two readings to be taken and subtracted one from the other.
- Measuring cylinder: To measure various volumes of liquids, accuracy depending on size and graduation of the cylinder (rather inaccurate).
- Beaker: To measure only approximate volumes of liquids, not to be used for precise quantities. It can be also used as a container.
- Conical flask: Used as a container.
- Evaporating dish: Used in crystallization.
- Volumetric flask: To prepare solutions with a specific volume, e.g. 250 cm3, 1000 cm3, etc., to the nearest 0.10 cm3.
- Test tube: Used for small-scale experiments.
- Boiling tube: To boil small quantities of solutions (this apparatus is very similar to the test tube with the only difference that its glass is thicker).
- Separating funnel: To separate two immiscible liquids.
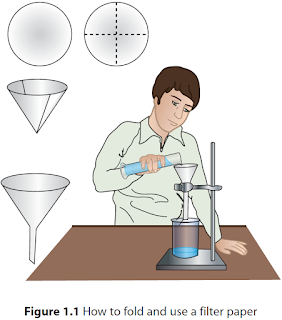
- Funnel: Used in filtration.
- Crucible tongs: To hold large hot chemical apparatus.
- Test tube holders: To hold test tubes while heating them.
- Thermometer: To measure the temperature.
- Wire Gauze: Allows uniform heat distribution when using a Bunsen burner.
Q1 Name the apparatus you would use to:
a. measure 25.6 cm3 of liquid.
Choose...
- Pipette
- Measuring cylinder
- Burette
b. measure 15 cm3 of liquid as accurately as possible.
Choose...
- Pipette
- Measuring cylinder
- Burette
c. measure approximately 100 cm3 of liquid.
Choose...
- Pipette
- Measuring cylinder
- Burette
answer :
a. measure
25.6 cm3 of liquid.
= Burette
b. measure
15 cm3 of liquid as accurately as possible.
= Pipette
c. measure
approximately 100 cm3 of liquid.
= Measuring cylinder
|
Q2 Arrange the following pieces of apparatus in the order of greatest accuracy first:
Measuring cylinder
Beaker
Burette
Pipette
1.2.2 Drawing chemical apparatus
When we draw chemical apparatus we usually show only cross sections. Do not attempt to draw three-dimensional diagrams or shade the drawings. It is important that diagrams are neat and tidy, that the pieces of apparatus are in proportion to one another and that everything is clearly labeled. The diagrams should be large enough to be seen clearly and should be in pencil. Use a ruler for straight lines and keep a set of coins for drawing circles.
Below is some of the apparatus we will be using in grade ten. You should be able to draw, label and identify each piece of apparatus and know its function.
Q3 Name the following glass articles which are usually found in a laboratory.
Choose...
- Pipette
- Funnel
- Conical flask
- Beaker
Choose...
- Pipette
- Funnel
- Conical flask
- Beaker
Choose...
- Pipette
- Funnel
- Conical flask
- Beaker
Choose...
- Pipette
- Funnel
- Conical flask
- Beaker
Steps to follow for lighting a burner
Before using the Bunsen burners in the lab, the teacher will open the main safety valve which would allow gas to come into the pipes (normally this will be closed)
a) Place the Bunsen burner on a porcelain tile to protect the surface of the bench.
b) Make sure that the Bunsen burner is attached to a gas tap.
c) Make sure that the air hole(s) of the Bunsen burner is/are closed.
d) Hold a lighted match or splint to the muzzle of the Bunsen burner and slowly open the gas tap until the gas ignites (Do not open the gas tap first, and keep your face and hair away from the burner). This will produce a very sooty, yellow flame.
e) Slightly open the air holes until the flame is blue at the bottom and slightly yellow at the top (the teacher will demonstrate first).
f) If a very hot flame is required, fully open the air hole and the gas tap. Be very careful with this type of flame and never leave the Bunsen burner with a blue flame when not
a) Place the Bunsen burner on a porcelain tile to protect the surface of the bench.
Why should you never leave the Bunsen burner with a blue flame when not in use?
There are two reasons for the last rule. First, a flame yellow at the top is more easily seen than a faint blue flame. Secondly, a blue flame is more likely to go off, allowing unburned gas to flow into the laboratory, which is not only a health hazard, but may cause an explosion.
Remember the following safety rules when using a Bunsen burner:
a) Do not open the gas tap before you place the lighted match near the muzzle of the burner.
b) Never leave the Bunsen burner with a blue flame when not in use.
Q4 Fill in the blank.
You should not open the tab before lighting a match to avoid any _______ in the laboratory.
Q5 Fill in the blank.
Never leave the Bunsen burner with a flame, when not in use.
Q6 Fill in the blank.
A blue flame is less visible and more likely to go off than a
flame.
1.2.4 Heating a test-tube
Observe the following rules when heating a test-tube:
1. Never have the test-tube more than half filled with liquid, otherwise the liquid may boil and spill out, causing bodily harm or putting out the flame.
2. Always use test-tube holders and never tongs or your hand. Tongs may cause the tube to slip out or break.
3. Do not hold the test tube upright in the flame, instead have it inclined at an angle as shown below. An upright tube is more likely to spit the liquid out when the liquid inside it starts to boil.
4. Keep the test-tube moving as it is being heated, by moving your wrist from side to side. This will help to prevent ‘bumping’ due to the liquid overheating at one specific spot (local overheating).
5. When the liquid starts to boil vigorously remove the tube temporarily out of the flame, or reduce the flame, so the liquid will not spill out.
Q7 Which of the following should be done while heating a test-tube?
Always use test-tube holders.
When the liquid starts to boil vigorously, keep the tube for a while on the flame.
Keep the test-tube still as it is being heated.
Never have the test-tube more than half filled with liquid.
Always hold the test tube upright in the flame for maximum heating.
1.2.5 Heating a beaker or evaporating Dish
Rules for heating a beaker or an evaporating dish:
a) The beaker or dish must be placed on a tripod and gauze as shown below.
b) Do not more than half-fill the beaker or dish with liquid.
c) Use tongs and never your hands to transfer the hot evaporating dish to a steam bath.
1.2.6 Crystallization
Crystallization is the process of forming crystals. Crystals are solid “objects” with distinctive plane faces at specific angles with respect to each other, angles that are characteristic of the crystalline material. Your teacher will show you a few samples of crystals.
Q8 Fill in the blank.
Q9 Fill in the blank.
1.2.6 Crystallization – Cont.
Crystallization can take place by cooling a pure liquid, as in the case of ice crystals forming when pure water is cooled. It can also take place by heating a solution (e.g. sea water) until the liquid evaporates and solid salt crystals remain. It can also take place by evaporation. When sugar is dissolved in water, and the water is allowed to evaporate, sugar crystals appear. Sea salt is obtained from the sea by collecting sea water in flat beds and allowing the warmth of the sun to evaporate the water.
In order to obtain crystals, the solvent (the liquid in which a solid is dissolved) has to be carefully evaporated in the final stages.
The technique involves the following steps:
a) Heat the evaporating dish directly with the Bunsen burner until most of the water has evaporated. Do not boil dry.
b) Now, using tongs, transfer the evaporating basin to a steam bath as shown below crystals?
Note: If only a small volume of salt solution is to be evaporated (25cm3 or less) then step (a) can be omitted.
Home experiment: In order to obtain good, larger crystals of a salt or sugar, dissolve sugar or table salt in some water, until no more can dissolve. Filter the solution by pouring it through two layers of kitchen paper (see filtration later in this chapter). Place the solution in a clean glass, covered with a light sheet of paper. Wait a few days. Bring the crystal to school. Who has managed to obtain the largest crystals?
Q 10What are the two conditions that should be taken into consideration while performing a crystallization to obtain large crystals of salt?
the solution must be mixed with another solvent
the solution should be saturated
the crystallization should take place very slowly
the solution must be heated quickly at very high temperature
Q11 Which of the following are procedures used for obtaining crystals?
- cooling a pure liquid
- evaporation
- heating a solution
- condensation
1.2.7 Units of volume in the laboratory
As you will soon see, many of the instruments we use in the laboratory are used to measure volume. In the laboratory, volume is usually measured in liters or in milliliters. The units that are used can be any of the following
Fill in the blank.
The units of volume used in the laboratory are and milliliters.
liters